Passeo-18 Lux
Vascular Intervention // Peripheral
Drug-Coated Balloon Catheter/0.018''/OTW
Clinically proven
Randomized controlled trials and all-comers registries have investigated safety and efficacy in the treatment of over 1,900 patients with peripheral artery disease (PAD) in the femoropopliteal and infrapopliteal arteries.
Safe and effective
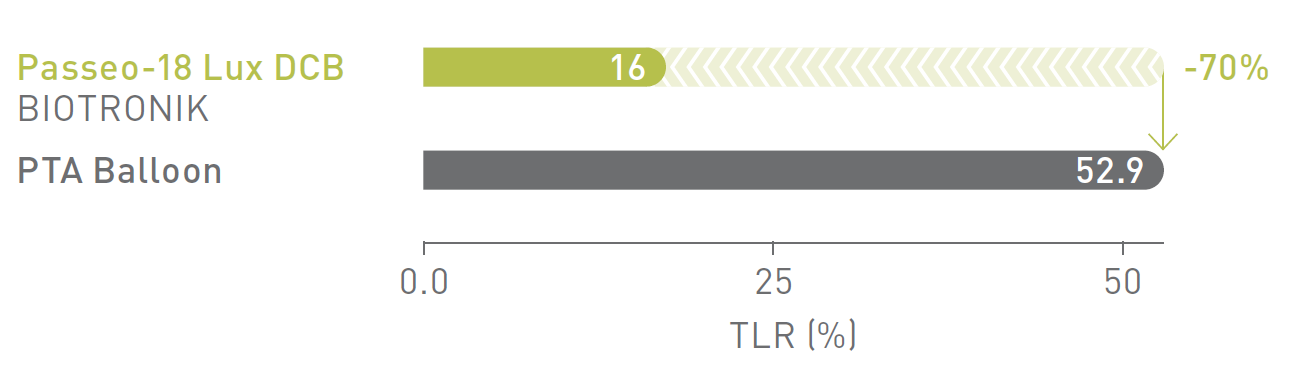
BIOLUX P-I RCT1 Femoropopliteal Indication
12-month Target Lesion Revascularization (TLR)
Passeo-18 Lux DCB significantly reduced TLR rates compared to the control PTA* balloon in the as-treated population.
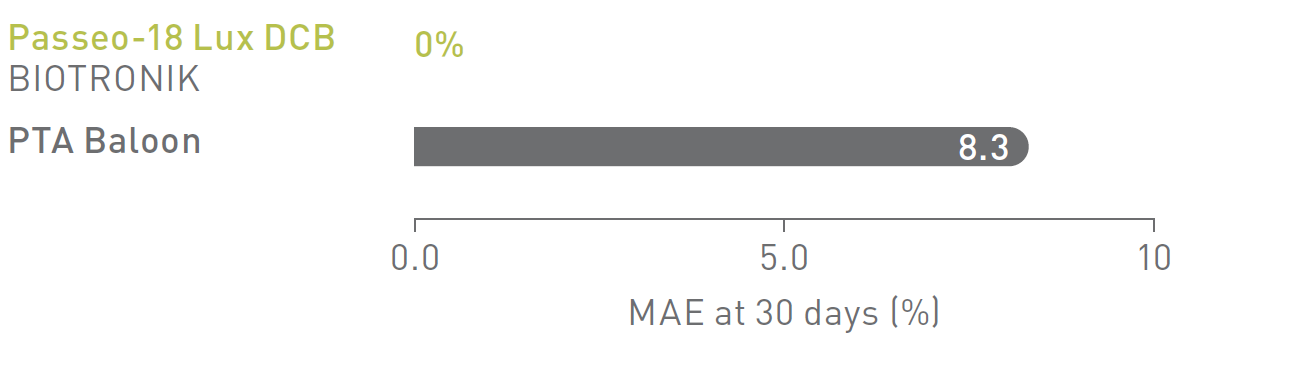
BIOLUX P-II2 Infrapopliteal Indication
Major Adverse Events (MAE)
MAE rate of the Passeo-18 Lux DCB was lower compared to the control PTA balloon.
BIOLUX P-III2 All-Comers Registry
Passeo-18 Lux DCB demonstrates excellent outcomes in one of the largest real-world DCB registries with few exclusion criteria.
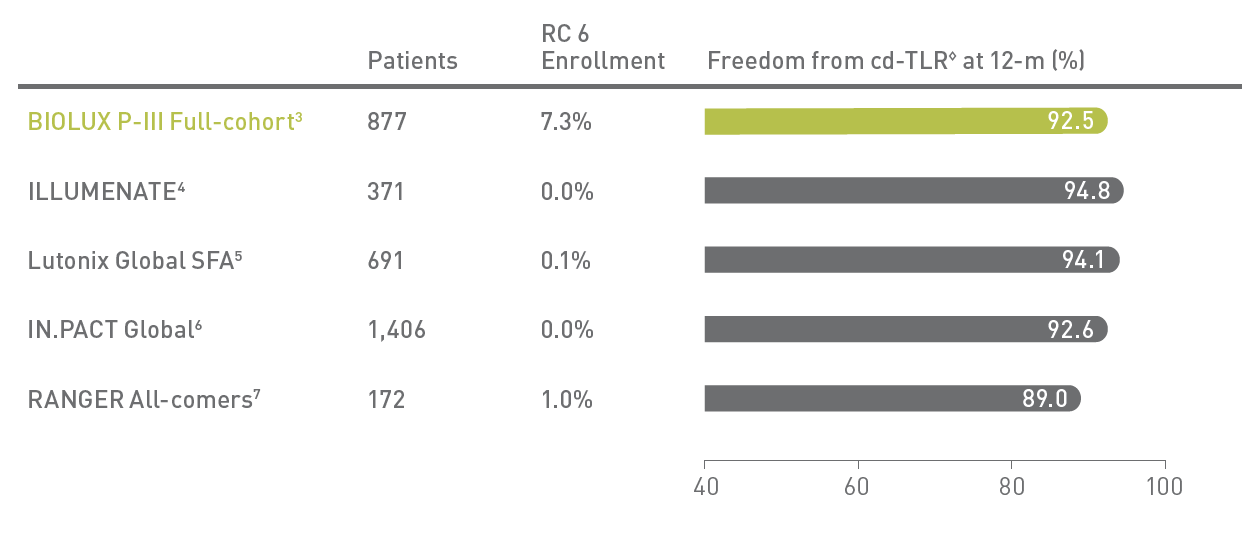 ◊Kaplan-Meier estimates; RC - Rutherford Classification; cd-TLR - clinically driven Target Lesion Revascularization.
◊Kaplan-Meier estimates; RC - Rutherford Classification; cd-TLR - clinically driven Target Lesion Revascularization.

For challenging patient groups
Safety and efficacy clinically proven across challenging subgroups in BIOLUX P-III all-comers registry
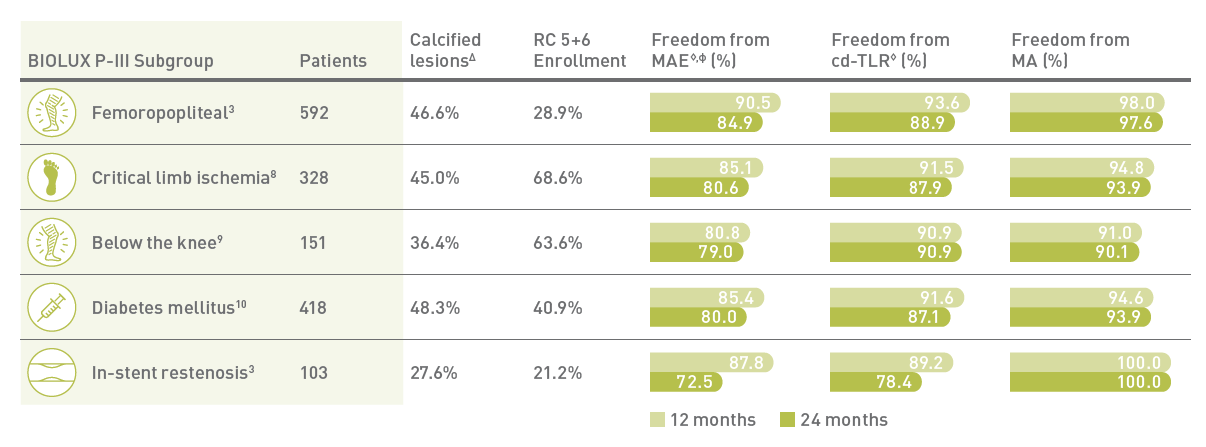 MA - Major target limb Amputations; Δ Moderate/Severe Calcified Lesions; ф Defined as composite of device - and procedure-related mortality through 30 days, and major target limb amputation and clinically driven target lesion revascularization.
MA - Major target limb Amputations; Δ Moderate/Severe Calcified Lesions; ф Defined as composite of device - and procedure-related mortality through 30 days, and major target limb amputation and clinically driven target lesion revascularization.

Effective drug delivery
Insertion and handling
The SafeGuardTM insertion aid improves ease of handling, and protects the user and balloon coating from contact and damage. It comes pre-mounted on the balloon and, after insertion, can simply be retracted and peeled away.
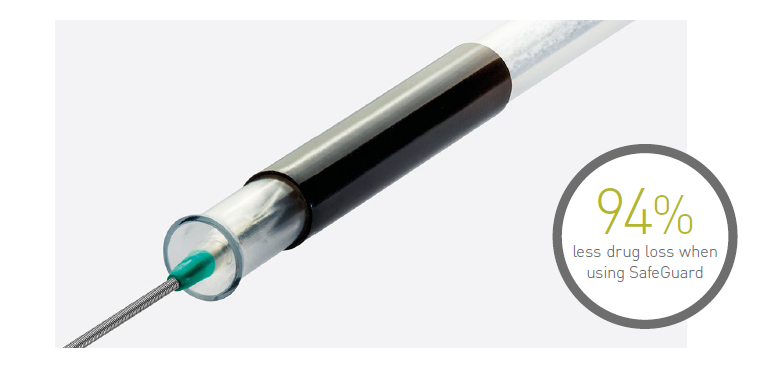
Reduction of drug loss in the introducer sheath valve11

High drug retention11
BIOTRONIK's Lux® coating provides a hydrophobic butyryl-tri-hexyl citrate (BTHC) excipient, which is less soluble than hydrophilic alternatives, ensuring more drug is available at the lesion site.
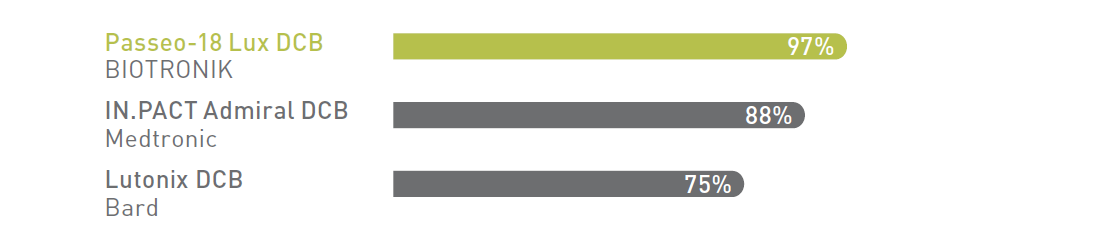 Drug coating integrity: % of drug load remaining on balloon after being submerged for ~90 seconds in physiological solution.
Drug coating integrity: % of drug load remaining on balloon after being submerged for ~90 seconds in physiological solution.
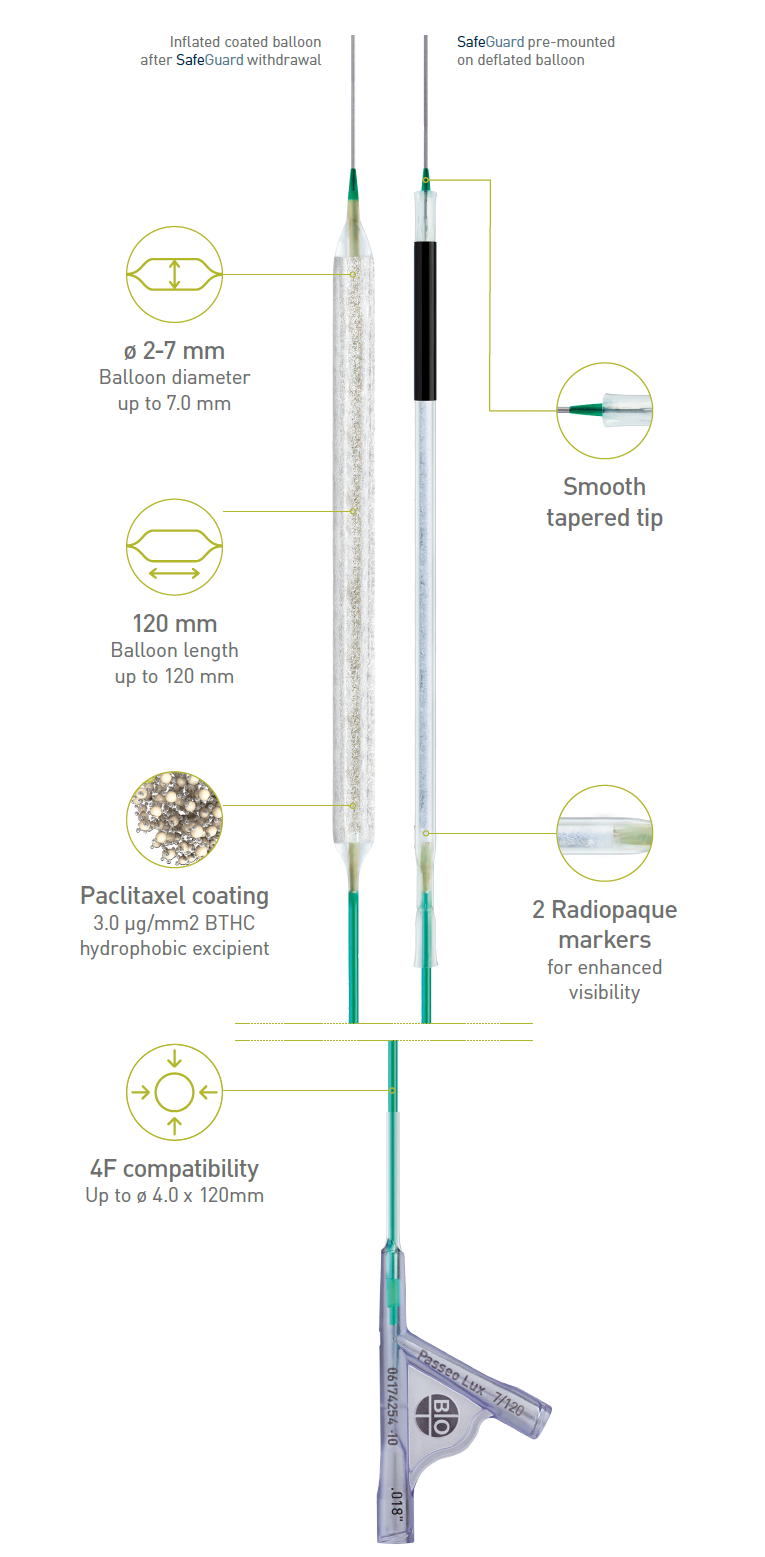
 Passeo®-18 Lux®
Passeo®-18 Lux®
Indicated to dilate de novo or restenotic lesions in the infrainguinal arteries.*
Technical Data
| Drug-coated balloon | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.018" |
| Tip | Short, tapered |
| Balloon markers | 2 swaged markers (zero profile) |
| Shaft | 3.8 F, hydrophobic coated |
| Usable length | 90, 130 cm; 150 cm (only ø 2.0 mm) |
| Introducer size | 4 F (ø 2.0 - 4.0 mm); 5F (ø 5.0 - 7.0 mm) |
| Nominal Pressure (NP) | 6 atm |
| Rated Burst Pressure (RBP) | 15 atm (ø 2.0 - 5.0 mm); 12 atm (ø 6.0 - 7.0 mm) |
| Coating | |
| Drug | Paclitaxel |
| Drug concentration | 3.0 μg/mm2 |
| Coating matrix | Paclitaxel and butyryl-tri-hexyl citrate (BTHC) |
| Coated area | Cylindrical section of the balloon, exceeding the proximal and distal markers |
Compliance Chart
| Balloon Diameter x Length (mm) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ø 2.0 x | ø 2.5 x | ø 3.0 x | ø 4.0 x | ø 5.0 x | ø 6.0 x | ø 7.0 x | |||||||||||||
| 40-120 | 40-120 | 40-120 | 40-120 | 40-120 | 40-120 | 40-120 | |||||||||||||
| Nominal Pressure | atm** | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |||||||||||
| (NP) | ø (mm) | 2.0 | 2.5 | 3.0 | 4.0 | 5.0 | 6.0 | 7.0 | |||||||||||
| Rated Burst Pressure | atm** | 15 | 15 | 15 | 15 | 15 | 12 | 12 | |||||||||||
| (RBP) | ø (mm) | 2.1 | 2.6 | 3.2 | 4.3 | 5.3 | 6.2 | 7.3 | |||||||||||
**1 atm = 1.013 bar
Ordering Information
| Catheter Length (cm) | Balloon ø (mm) | Balloon Length (mm) | |||
|---|---|---|---|---|---|
| 40 | 80 | 120 | |||
| 4F | 90 | 2.0 | 379860 | 379861 | 379862 |
| 90 | 2.5 | 379866 | 379867 | 379868 | |
| 90 | 3.0 | 370843 | 370848 | 370853 | |
| 90 | 4.0 | 370844 | 370849 | 370854 | |
| 5F | 90 | 5.0 | 370845 | 370850 | 370855 |
| 90 | 6.0 | 370846 | 370851 | 370856 | |
| 90 | 7.0 | 370847 | 370852 | 370857 | |
| 4F | 150 | 2.0 | 379863 | 379864 | 379865 |
| 130 | 2.5 | 379869 | 379870 | 379871 | |
| 130 | 3.0 | 370858 | 370863 | 370868 | |
| 130 | 4.0 | 370859 | 370864 | 370869 | |
| 5F | 130 | 5.0 | 370860 | 370865 | 370870 |
| 130 | 6.0 | 370861 | 370866 | 370871 | |
| 130 | 7.0 | 370862 | 370867 | 370872 | |











 Temporary pacemakers
Temporary pacemakers Permanent pacemakers
Permanent pacemakers Temporary pacemaker leads
Temporary pacemaker leads Home Monitoring
Home Monitoring  Instruments
Instruments