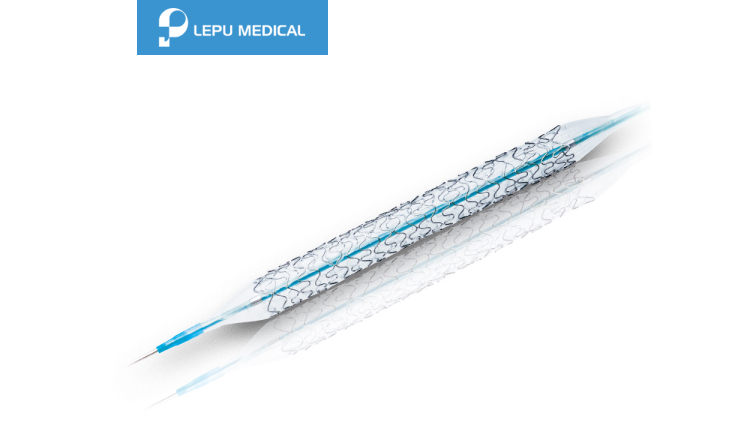
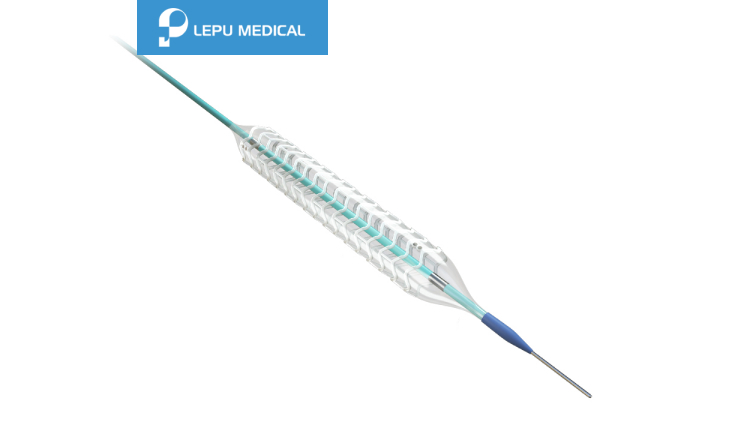
Nano+™
Nano+™ Polymer-free Sirolimus-eluting Coronary Stent System Details
Product Description
-
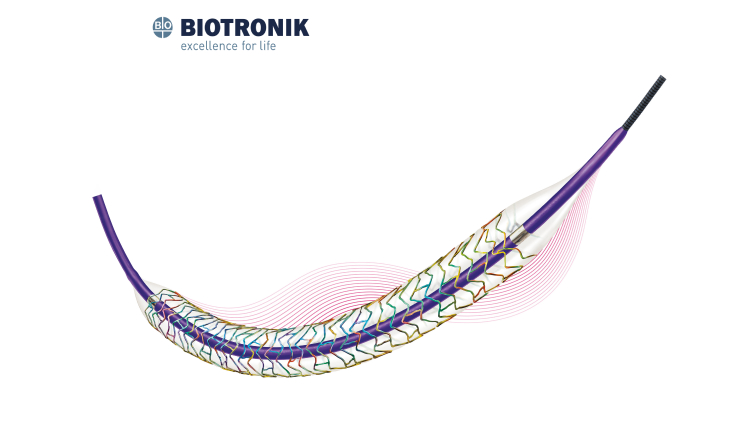
Nanoporous cavities on strut abluminal surface functioning as drug carrier to ensure the firm adhesion.
-
Vacuum drug coating technology ensures complete drug dosage and coating stability.
-
Post-treatment after drug loading helps with sustained drug release.

-
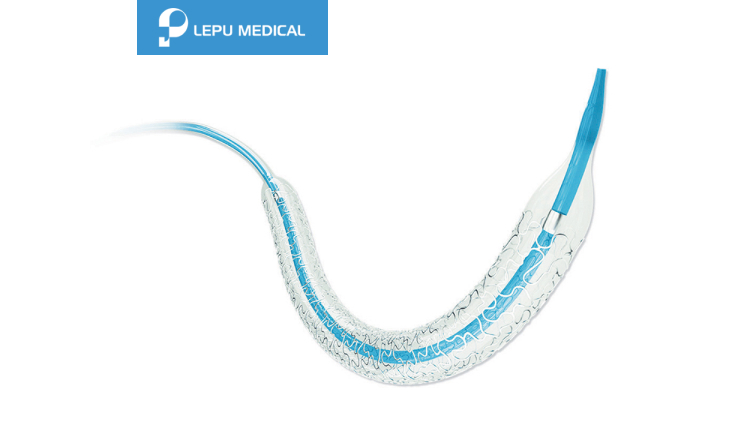
Soft tapered tip provides smooth transition from guidewire to balloon catheter and excellent crossability.
-
Short shoulder design at both ends of the balloon ensures the accurate dilation to the lesion, and reduces the damage to normal vessel next to the lesion.
-
Patented hydrophilic coating on distal shaft provides outstanding pushability.

-
Polymer-free design significantly reduces the inflammatory response after stent implantation to guarantee the long-term safety.
-
Polymer-free design facilitates earlier endothelialization which significantly shorten the DAPT time.
-
The rough strut surface helps with earlier and easier endothelialization to reduce the risk of thrombosis.
DES Efficacy [1]

BMS Safety [2]

Nano+™ OCT Study
An OCT Study in Europe Shows Nano+™ A Great Combination of DES Efficacy and BMS Safety

Prouduct Features
-
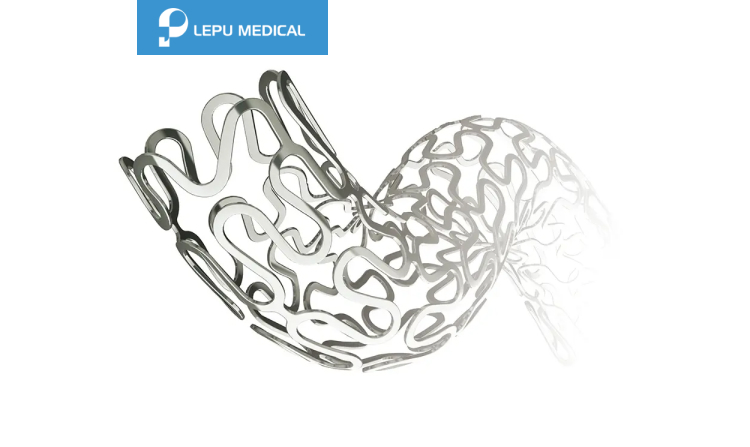
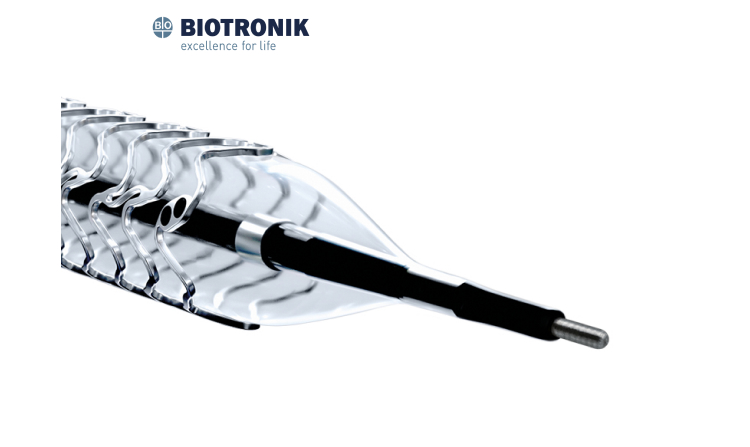
Polymer-free DES
-
Nanoporous drug carrier on abluminal surface
-
Balanced strut thickness
-
High radial force
-
Sine wave + 3-3-3 link
-
Double helix structure
-
Large open cell
-
Precise positioning markers
-
Effective drug of Sirolimus
-
Vacuum drug coating technology
-
90-day drug releasing period
-
New delivery system
Prouduct Benefits
-
Significantly shorten DAPT time
-
Earlier & better endothelialization
-
Highly reduced risk of thrombosis
-
Great deliverability
-
Easy positioning and placement
-
Optimal tissue scaffolding
-
Excellent side branch access
-
Scientific drug releasing period
Prouduct Ranges
-
Ø 2.5 - 4.0 mm
-
12 - 36 mm multi stent lengths
Clinical Studies
Specifications
Ordering Information
An OCT Study in Europe Shows Nano+™ A Great Combination of DES Efficacy and BMS Safety [3].
In a prospective, multicentre, single-arm, open-label study, OCT examinations
were performed at three months in 45 patients (47 lesions). 23 patients with 24 lesions
had serial coronary angiography and OCT assessment at three and six months.
OCT Analyses
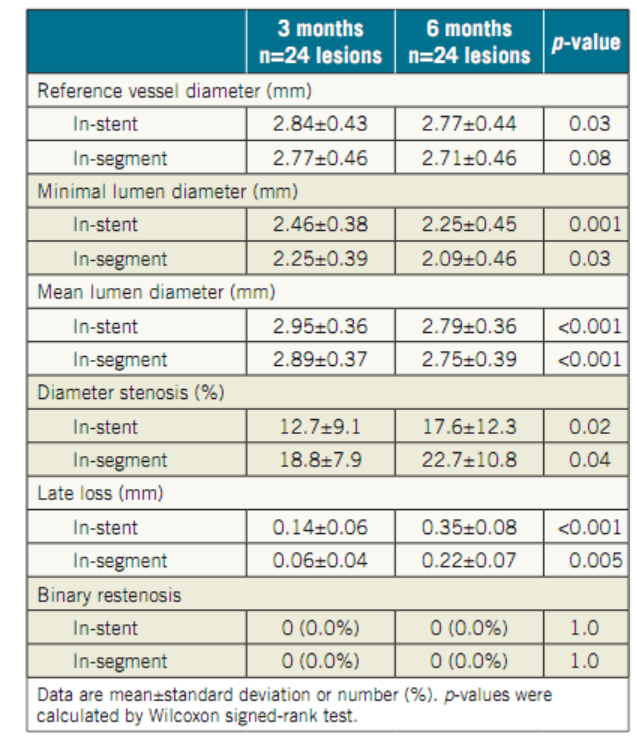
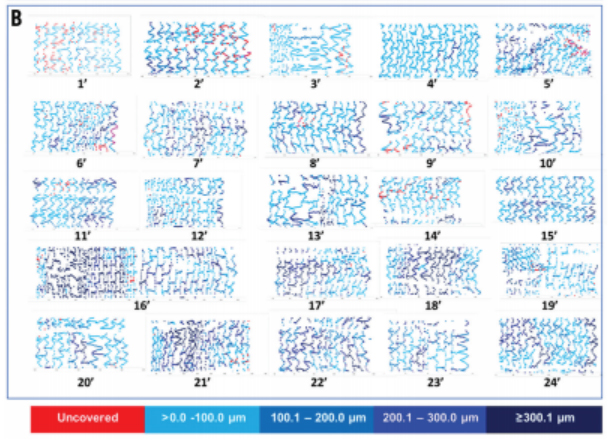
Coverage status of the individual stents/patients at two time points (three and six months).
Serial OCT showed almost complete vascular healing at six months, even when coverage was insufficient at three months. This suggests an adequate safety and efficacy profile of the device at that point in time.
















.png) Diagnostic catheters
Diagnostic catheters.jpg) Guidewire
Guidewire.jpg) Micro-catheters
Micro-catheters Guiding catheter
Guiding catheter